Compliance with Chapter 4 “Documentation” of the EU Good Manufacturing Practice (GMP) is one of the most critical and complex aspects for companies in the pharmaceutical and manufacturing sectors. This regulatory section requires that all documentation related to production processes, quality control, and inspections be accurate, accessible, and above all immutable, to ensure traceability, safety, and compliance during audits and certifications. Certiblok positions itself as a cutting-edge solution that comprehensively meets these regulatory needs, offering blockchain-based digital document management that guarantees unalterability and full authenticity.
Traditional document management—often still based on paper archives or centralized digital systems—frequently presents issues related to errors, missing documents, or untracked changes. These risks expose companies to potential penalties, delays in certification processes, and difficulties in managing internal and external audits. The blockchain technology at the core of Certiblok leverages unique features such as decentralization and cryptography to create distributed, immutable ledgers. Every document uploaded to the platform is transformed into a cryptographic “fingerprint” that certifies its date, content, and ownership, with no possibility of alteration.
This immutability is crucial for meeting the ALCOA+ principles (data attributes that ensure quality, accuracy, and accountability) underpinning European GMPs, where every datum must be attributable, legible, contemporaneous, and accurate, and it must be possible to demonstrate total integrity throughout its lifecycle. Certiblok not only preserves documents but also manages versions and revisions, automatically notifying stakeholders of updates and preventing the circulation of obsolete or unauthorized versions.
The platform also ensures data security against unauthorized access and cyberattacks—an essential factor for companies subject to such stringent compliance standards. Decentralized storage reduces the risk of document loss or compromise, enabling operational continuity even in IT crisis scenarios. Thanks to an advanced permissions and access-control system, only authorized users can view or modify documents, while maintaining a complete audit trail of every operation performed.
Another advantage of Certiblok is the “Audit Room,” a feature that allows you to create secure digital spaces shared with auditors, regulatory bodies, and commercial partners, speeding up inspections and significantly reducing the time needed to provide evidence and timely responses to inspection requests. This digital interactivity, combined with the assurance provided by blockchain, transforms audits from a critical and often stressful moment into a smooth, controlled process.
From an operational standpoint, Certiblok integrates effectively with existing IT infrastructures in pharmaceutical or manufacturing companies, enhancing document-management tools with the security and transparency guarantees typical of blockchain. This makes the solution not only a regulatory response but also an enabling element for more efficient, modern digital governance.
We also offer the possibility to integrate Certiblok with all existing ERPs in the company via REST API, preserving operators’ usual activities while harnessing Certiblok’s power and security for document management.
Managing documentation in accordance with EU Good Manufacturing Practice (GMP) Chapter 4 can be particularly challenging for companies. With its cryptographic security, immutable traceability, and streamlined digital collaboration, the Certiblok platform stands out as an indispensable tool to ensure regulatory compliance, improve process efficiency, and reduce risks for regulated businesses.
You can discover all the platform’s benefits directly on the Certiblok features page, or explore practical examples and case studies on our official blog.
Compila il form qui sotto per richiedere il Piano FREE o il Piano BUSINESS completo per 30 giorni
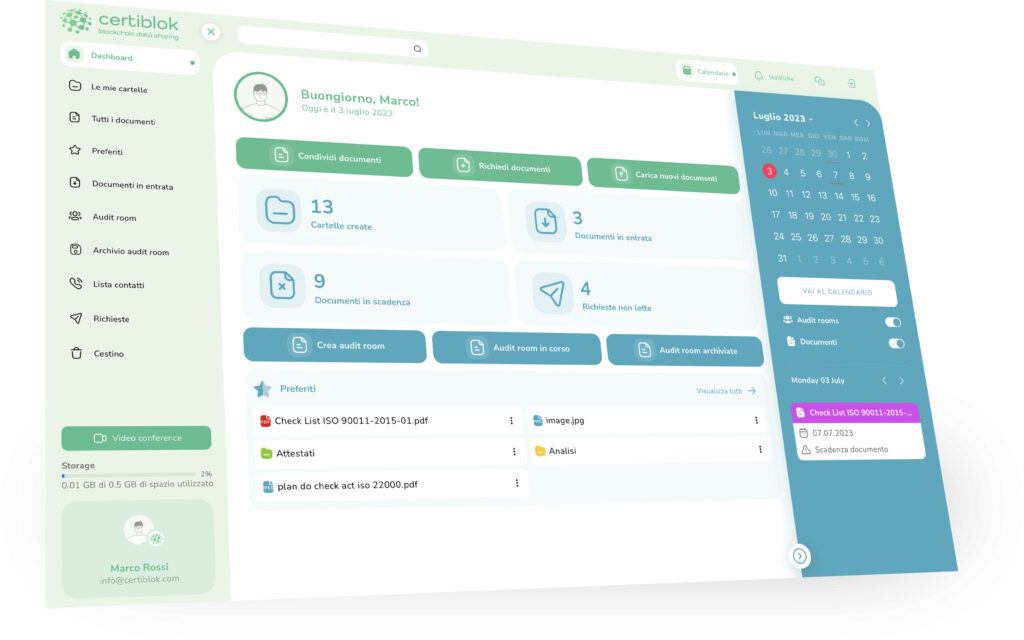
CERTIBLOK, LA PIATTAFORMA DRM® Document Relationship Management,
che rivoluziona il modo di gestire e condividere i documenti, anche quelli più riservati. Semplifica il lavoro in team, gestisce le scadenze, ti collega con clienti, fornitori, consulenti ed enti ispettivi, garantendo la massima protezione del tuo patrimonio documentale attraverso il cloud decentralizzato e la tecnologia Blockchain.Funzioni